| Soundings - copied from TNAUK website
Talking
Newspaper Association of the UK - TNAUK website
How the eye
works - copied from TNAUK website, originated from Optobionics
Corporation
[25th
Anniversary Celebrations]
[Cassette
Recorders]
[History]
[Information Tapes]
[Library]
[Links
and Downloads]
[News]
[News2]
[Newsletter] [Newspapers and
Magazines] [Pendle Residents] [TNAUK] [UK
Residents
[Home]
|
|
BT
Soundings - www.visound.org/btsoundings.html
- The UK's audio magazine for blind and partially sighted people. This
free monthly cassette tape offers impartial, public service information
that is of particular interest to anyone with poor or failing sight.
More details by calling 01273 303111 or by emailing mailto:btsoundings@visound.org
______________________________________________
The
Talking Newspaper Association of the UK (TNAUK) is a
registered charity which provides national and local newspapers and
magazines on audio tape, computer disk, e-mail and CD-ROM for visually
impaired and disabled people who find reading a strain. Local
newspapers and magazines are supplied free of charge by over 520 local
talking newspaper groups which are affiliated to TNAUK but are
autonomous.The national service is provided by the TNAUK headquarters in
Heathfield, East Sussex. For a nominal fee of £25, subscribers have a
choice of 10 publications from over 200 titles. A 'Guide to Tape
Services for Visually Impaired and Disabled People' is available from
TNAUK in print and on disk for £7 and contains details of local talking
newspaper groups, other information available on tape and organisations
for visually impaired and disabled people
______________________________________________
How The
Eye Works
Our ability to see is the result of a process very similar to that of a
camera. With a camera, light rays pass through a series of lenses which
focus images onto film. The eye performs a similar function in that
light rays pass through the cornea and crystalline lens which focus
images onto the retina. The retina is a layer of light sensing cells
which line the back of the eye.
The area of the retina that receives and processes the detailed images
and then sends them via the optic nerve to the brain is referred to as
the macula. The macula is of significant importance in that this area
provides the highest resolution for the images we see. The macula is
comprised of multiple layers of cells which process the initial
"analog" light energy entering the eye into
"digital" electro-chemical impulses.
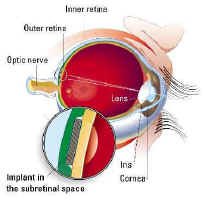
picture of the human eye showing where the
Artificial Retina will be placed. Click to see it large
What Is RP or RETINITIS PIGMENTOSA
RETINITIS PIGMENTOSA (RP) is a general term for a number of diseases
that predominately affect the photoreceptor layer or "light
sensing" cells of the retina. These diseases are usually hereditary
and affect individuals earlier in life.
Injury to the photoreceptor layer, in particular, reduces the retina's
ability to sense an initial light signal. Despite this damage, however,
the remainder of the retinal processing cells in other layers usually
continue to function.
Although different forms of RP may affect
different specific areas of the visual field, most RP affects the
mid-peripheral vision first and, sometimes, progresses to affect the
far-periphery and the central areas of vision.
The narrowing of the field of vision into "tunnel vision" can
sometimes result in complete blindness. Some specific forms of RP and
related conditions include Usher's Syndrome, Leber's Congenital
Amaurosis, Stargardt's Disease, Cone-Rod Dystrophy, Best's Disease, and
Choroideremia, and Gyrate Atrophy.
AGE-RELATED MACULAR DEGENERATION (AMD) refers to a degenerative
condition that occurs most frequently in the elderly.
AMD is a disease that progressively decreases the function of specific
cellular layers of the retina's macula. The affected areas within the
macula are the outer retina and inner retina photoreceptor layer.
Patients with macular degeneration experience a loss of their central
vision, which affects their ability to read and perform visually
demanding tasks. Although macular degeneration is associated with aging,
the exact cause is still unknown.
Together, AMD and RP affect at least 30 million people in the world.
They are the most common causes of untreatable blindness in developed
countries and, currently, there is no effective means of restoring
vision.
The Artificial Silicon Retina (ASR) was invented by brothers Alan and
Vincent Chow, who are also the co-founders of Optobionics Corporation.
The ASR is a silicon chip 2 mm in diameter and 1/1000 inch in thickness.
It contains approximately 3,500 microscopic solar cells called "microphotodiodes,"
each having its own stimulating electrode. These microphotodiodes are
designed to convert the light energy from images into thousands of tiny
electrical impulses to stimulate the remaining functional cells of the
retina in patients with AMD and RP types of conditions.
The ASR is powered solely by incident light and does not require the use
of external wires or batteries. When surgically implanted under the
retina, in a location known as the subretinal space, the ASR is designed
to produce visual signals similar to those produced by the photoreceptor
layer. From their subretinal location these artificial
"photoelectric" signals from the ASR are in a position to
induce biological visual signals in the remaining functional retinal
cells which may be processed and sent via the optic nerve to the brain.
In preclinical laboratory testing, animal models implanted with the ASRs
responded to light stimuli with retinal electrical signals (ERGs) and
sometimes brain-wave signals (VEPs). The induction of these biological
signals by the ASR indicate that visual responses had occurred.
In collaboration with the Hines Veterans Administration Medical Center,
the Louisiana State University Eye Center, the University of Illinois
Eye Center in Chicago, Stanford University's Nano Fabrication Facility,
and Tulane University Medical Center, Optobionics has been researching
and developing means to further improve the biocompatibility and
function of the ASR.
The first clinical trials in human beings began On June 30, 2000.
Clinical Tests
JUNE 30, 2000 In landmark surgeries at the University of Illinois at
Chicago Medical Center on June 28, and at Central DuPage Hospital,
Winfield, Illinois on June 29, the first artificial retinas made from
silicon chips were implanted in the eyes of three blind patients who
have lost almost all their vision from retinitis pigmentosa. All three
patients were released from the hospital the following day. Preliminary
tests have determined that no complications have occurred.
The surgical team for all three operations consisted of Drs. Alan Chow,
President and CEO of Optobionics Corporation of Illinois, the company
that invented and developed the artificial retina, Gholam Peyman,
Professor of Ophthalmology and Co-Director of Vitreoretinal Surgery at
Tulane University Medical Center, and Jose Pulido, Professor and Head of
the Department of Ophthalmology and Visual Sciences at the University of
Illinois at Chicago. "We've completed the first part of our journey
to the Holy Grail of restoring eyesight to the blind," Dr.
Pulido said.
The Artificial Silicon Retina (ASR) was invented by Dr. Alan Chow and
his brother Vincent Chow, Optobionics' Vice President of Engineering. It
is a silicon microchip 2mm in diameter and one-thousandth of an inch
thick less than the thickness of human hair. Preliminary laboratory
studies were performed in conjunction with Dr. Neal Peachey and his
research group at the Edward Hines, Jr. VA Hospital in Chicago.
The ASR contains approximately 3,500 microscopic solar cells that
convert light into electrical impulses. The purpose of the chip is to
replace damaged photoreceptors, the "light-sensing" cells of
the eye, which normally convert light into electrical signals within the
retina.
Loss of photoreceptor cells occurs in persons with retinitis pigmentosa
(RP) and other retinal diseases. All three patients who received the
implants have lost almost all their vision from retinitis pigmentosa.
The two men and one woman, two of whom use guide dogs, are between 45
and 75 years of age.
The landmark surgeries performed in the two area hospitals were part of
a feasibility and safety study approved by the Food and Drug
Administration to determine whether the ASR could be safely implanted
and tolerated in the human eye. "In this study, we are evaluating
the safety and feasibility of the ASR by placing a small version of the
implant in a side portion of the retina. The operations to place the
implants in this location were all successfully completed. We hope that
if the implants are able to stimulate the retina, patients may develop
some degree of vision over the location of the implant within the next
month," said Dr. Chow.
The microsurgical procedure starts with three tiny incisions in the
white part of the subject's eye, each incision no larger than the
diameter of a needle. Through these incisions, the surgeons introduce a
miniature cutting and vacuuming device that removes the gel in the
middle of the eye and replaces it with saline. They then make a pinpoint
opening in the retina through which they inject fluid to lift up a
portion of the retina from the back of the eye, creating a small pocket
in the "subretinal space" just wide enough to accommodate the
ASR. The surgeons then enlarge the pocket opening and insert the implant
into the subretinal space. Finally, they reseal the retina over the ASR,
introduce air into the middle of the eye to gently push the retina back
down over the device, and close the incisions. Over a period of one or
two days, the air bubble is resorbed and replaced by fluids created
within the eye.
According to Dr. Peyman, "The use of the subretinal space to hold a
device that artificially stimulates the retina seems a logical step in
replacing the loss of photoreceptor cells of the retina. If the implant
is tolerated well and is able to successfully stimulate the retina, it
may open up new opportunities for restoring sight in patients with the
end stages of retinitis pigmentosa."
Note To Patients
The ASR is designed to interface and function with a retina that has
partial outer retinal degeneration. This means that although the
photoreceptor cellular layer of the retina may be Degenerated outer
retinal photoreceptor cells damaged, the remaining cellular layers are
still functional. This is most commonly associated with conditions such
as RP and AMD.
Specific and related conditions which may possibly be amenable to
treatment with ASRs include some forms of long-term retinal detachment,
Usher's Syndrome, Leber's Congenital Amaurosis, Stargardt's Disease,
Cone-Rod Dystrophy, Best's Disease, Choroideremia, Gyrate Atrophy, and
retinal diseases that specifically affect the photoreceptor layer but
spare the remaining inner layers of the retina. Whether these conditions
will actually respond to ASR treatment will only be shown by clinical
testing.
The ASR relies on the ability to stimulate the remaining functional
cells within a partially degenerated inner or neuro retina. As a result,
the ASR will not be able to assist patients with disease conditions
where the retina or components of the visual system beyond the
photoreceptor layer have been substantially damaged. Such conditions
include glaucoma, optic nerve diseases such as optic neuropathy and
optic neuritis, retinal artery or vein occlusions, diabetic eye disease
with severe retinal scarring, and blindness caused by stroke or other
injuries to the seeing part of the brain.
In retinopathy of prematurity (RLF and ROP), the ASR cannot stimulate
the retina if an irreparable "funnel detachment" of the retina
has occurred. Even if the retina can be surgically "flattened"
and lacks significant scarring, there is still only a very limited
possibility of vision improvement because of complications due to
amblyopia.
Amblyopia is caused when portions of the seeing part of the brain fail
to develop in the absence of signals of clear vision from the eyes. The
ASR's potential to provide improved vision is, therefore, much less
likely in cases of early vision loss (such as Leber's Congenital
Amaurosis) compared with late vision loss types of RP. It is noted,
however, that some patients experience improved vision in an amblyopic
eye if, for some reason, vision in the better-seeing eye is lost thus an
amblyopic eye is sometimes considered to be a "backup" eye.
Macular holes are conditions that we have not evaluated closely and
cannot offer a definitive response to at this time.
TODAY, THE ASR IS STILL BEING DEVELOPED, AND IS NOT AVAILABLE FOR
TREATMENT OUTSIDE OUR CLINICAL TRIALS.
Frequently Asked Questions And Answers
QUESTION: What may a person be able to see with the ASR chip if it
proves successful in inducing vision?
ANSWER: The resolution capability of the ASR chip in blind persons will
not be known until clinical studies have progressed. Although it is
unlikely that a patient with the ASR chip will see images such as those
shown on the right, Optobionics is presently studying the effect of ASR
pixel density on image quality.
QUESTION: Other efforts are also underway to develop an artificial
retina. What is the difference between Optobionics' approach and these
other approaches?
ANSWER: Optobionics' Artificial Silicon Retina (ASR) may be used as a
stand-alone implant that is placed behind the retina (subretinal
approach) to directly stimulate the remaining viable cells of the
retina.
An array of microphotodiodes is fabricated on the chip so that the chip
itself converts light energy to electrical signals. These are designed
to directly stimulate the remaining overlying cells of the retina. We
understand other approaches utilize implants, positioned on the surface
of the retina (epiretinal approach), to try to stimulate the nerve-fiber
layer or ganglion cells.
We also understand that these other devices are designed to function in
conjunction with computers, video cameras, lasers, and/or radio
frequency transmitters. Thus Optobionics' subretinal approach differs
from the epiretinal approach in that:
(1) the design of our chip is relatively simple and may be able to
function solely with the power provided by light entering into the eye
presently our chip does not require connecting wires, batteries, or
other ancillary devices and :
(2) the placement of our chip is in contact with earlier processing
cells of the retina, so that some level of normal retinal processing of
images may be possible. This will hopefully allow the chip to generate
relatively-high quality images.
QUESTION: Can the ASR be used to treat animals?
ANSWER: At present time, the ASR is designed only for human use. There
are no plans to use the ASR in the treatment of animals with eye
diseases.
QUESTION: When will the ASR be available to the public? What must happen
between now and then?
ANSWER: This will depend on the results of the initial clinical trials
and possible modifications that may be required to provide an adequate
level of safety and efficacy.
QUESTION: What will the ASR cost?
ANSWER: The cost of the ASR has not been determined at this time.
[25th Anniversary
Celebrations] [Cassette
Recorders] [History] [Information Tapes] [Library]
[Links and Downloads] [News]
[News2]
[Newsletter] [Newspapers and
Magazines] [Pendle Residents] [TNAUK]
[UK Residents] [ Home ]
|